AG Hennig
The Hennig group investigates Drosophila dosage compensation as a model system to understand transcription and translation regulation by RNA-binding protein and long non-coding RNAs.
Dosage compensation is an essential molecular process in sexually reproducing organisms, which compensates the imbalance of number of sex chromosomes between the sexes. Although these processes can be quite different between species, recent research shows that all have common mechanisms. Messing with this cellular process has lethal consequences. In fruit flies, a well-established model organism, dosage compensation is regulated on the level of translation (females) and transcription (males). See Figure 1 for more details.
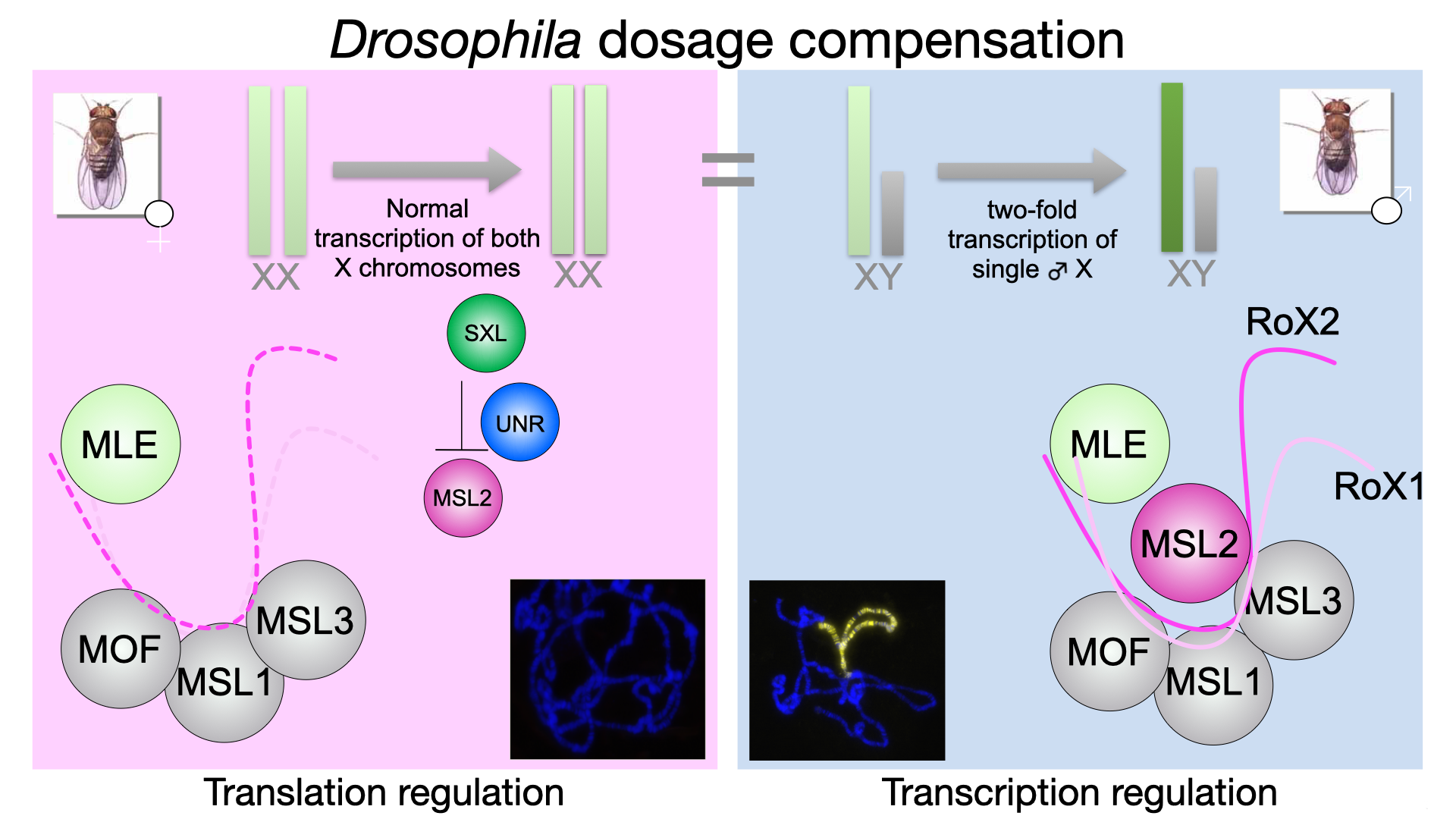
Figure 1: In males (right), the dosage compensation or male-specific lethal (MSL) complex, consisting of five proteins (RNA helicase maleless (MLE), MSL1-3 and acetyltransferase males-absent-on-first (MOF)) and two redundant long non-coding RNAs RNA-on-X 1 and 2 (roX1, roX2) binds with remarkable specificity only to the single male X-chromosome and achieve a two-fold hypertranscription of X-linked genes. This would be lethal in females (right). To prevent this, the female-specific protein Sex-lethal (Sxl) binds together with another RNA binding protein Upstream-of-N-Ras (Unr) to msl2 mRNA and prevents its translation. Thus, the MSL complex cannot be formed resulting in normal transcription of both female X chromosomes.
Male dosage compensation - transcription regulation
A prerequisite for the assembly of the MSL complex is the remodelling of lncRNA RoX2 (Figure 2A). RNA helicases are essential enzymes, which unwind or remodel RNAs. The RNA helicase MLE is needed to form an alternative RNA stem loop (ASL) to be incorporated into the MSL complex for targeting of the single male X chromosome. We could reveal the mechanism of the complete ATP-dependent helicase cycle by solving six cryo-EM structures of MLE at different states (Figure 2B, Jagtap et al., Mol Cell, 2023). Large conformational rearrangements unwind roX2 and make the formation of the ASL possible. We now want to understand how MSL2 binds to this RNA, allowing MSL complex assembly. Also, we do not know the role of MLE’s G-patch, which is another aspect of our current investigation.
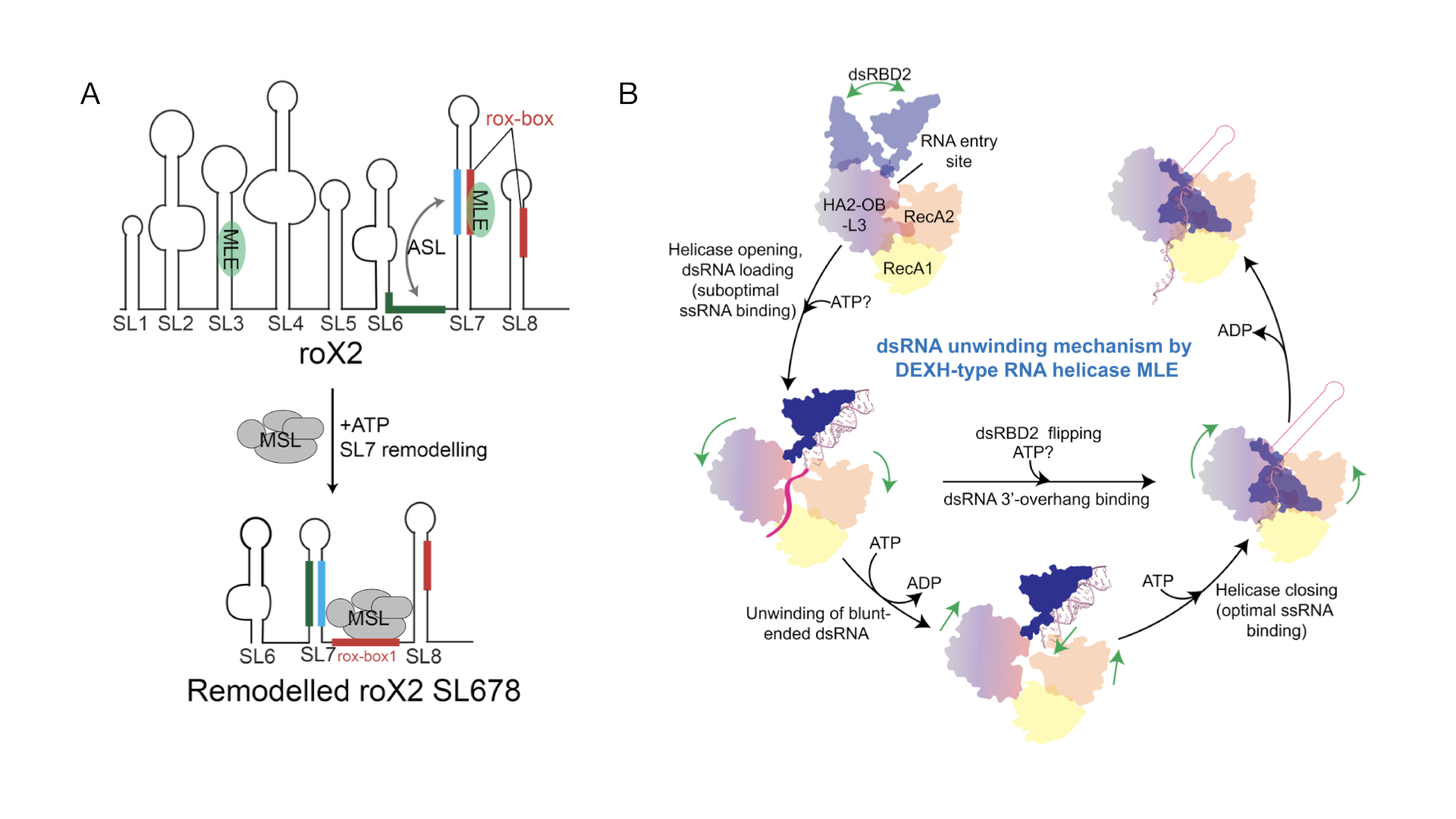
Figure 2: Remodelling of lncRNA RoX2 by MLE. A: RoX2 consists of eight RNA stem-loops of which stem loops (SL)678 is the essential part. MLE unwinds SL7, while the alternative stem loop (ASL) can be formed to allow binding of MSL2. B: the helicase cycle of MLE, starting with binding to double-stranded (ds)RNA, pulling the 3’end of the RNA into the core by conformational rearrangements triggered by ATP hydrolysis. The double-stranded RNA binding domain (dsRBD)2 then needs to bind to the helicase core to allow complete threading of the RNA through the RNA tunnel.
Why is this important for humanity?
DHX9 is the direct orthologue of MLE in humans. This RNA helicase is involved in many essential processes within human cells. It is highly overexpressed in many cancer cell lines where it supports proliferation by allowing fast translation. DHX9 has the same domain arrangement as MLE and it is thus very likely that DHX9 acts in a similar way. We are currently investigating the druggability of a novel interface of DHX9, which would allow highly specific targeting with small molecules. Preliminary results are very promising.
Female dosage compensation – translation regulation
We contributed significantly to understand the structural basis of why msl2 mRNA translation is repressed in females, which prevents hypertranscription of female X chromosomes (Hennig et al., Nature, 2014). The female-specific RNA-binding protein Sxl binds cooperatively with Unr to a specific sequence in the 3’untranslated region of msl2 mRNA (Figure 3A). Together with a third factor, Hrp48, this protein-RNA complex prevents the 43S preinitiation complex to bind. Thus, the ribosome cannot form to initiate translation at the start codon. We have solved more structures of Unr and of Unr in complex with the poly(A)-binding protein (pAbp, Hollmann et al., Cell Rep., 2020, Hollmann et al., Nucleic Acids Res, 2023, Figure 3B). Although, this revealed highly interesting properties of Unr with regards to RNA binding and more, the exact mechanism of this translation repression complex remains unknown. We will find out more by using NMR, cryo-EM and TRIF microscopy for smFRET measurements.
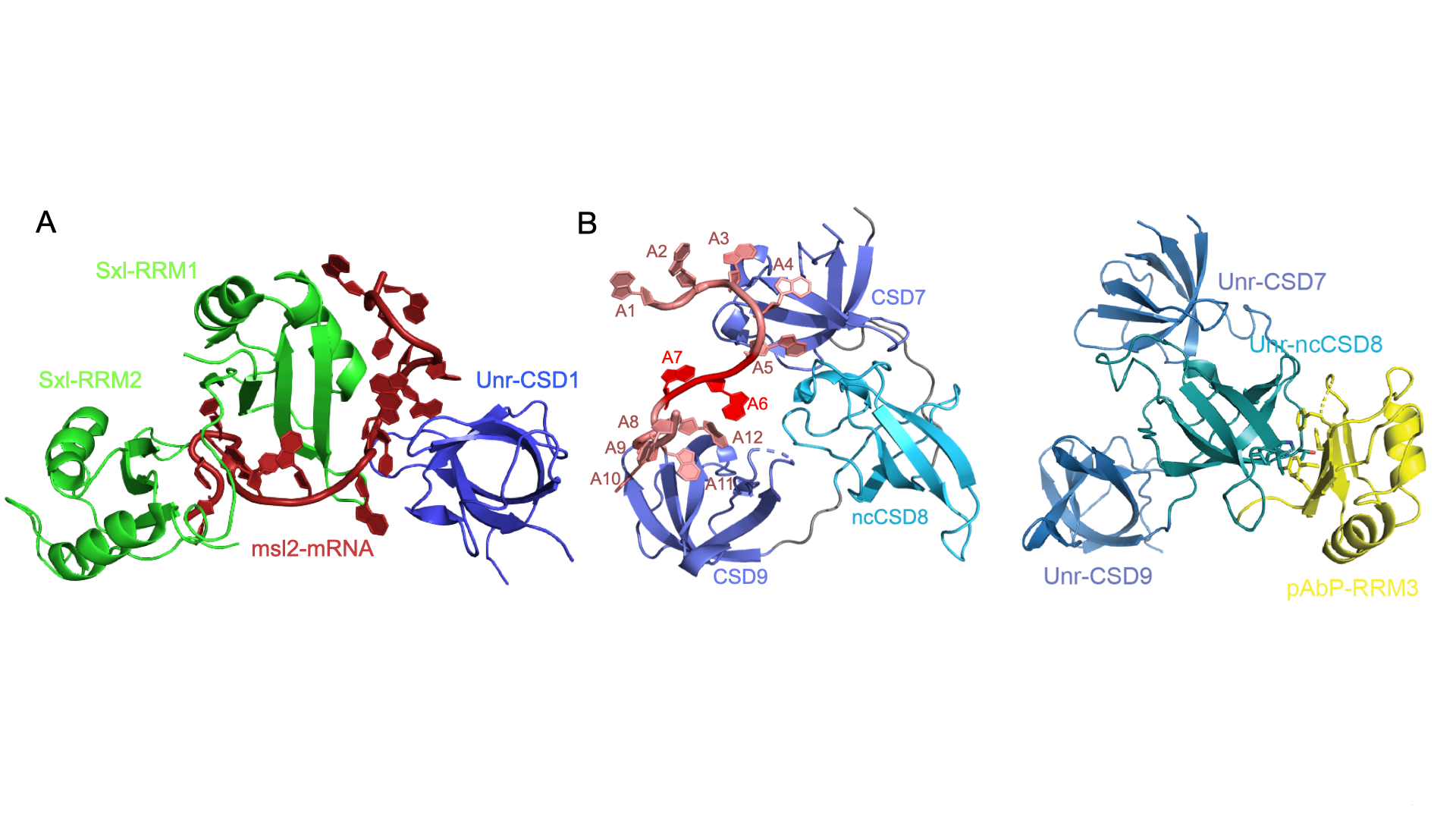
Figure 3: Sub-complexes of the msl2-mRNA translation repression complex. A: The essential core of the complex formed by Sxl and Unr binding cooperatively to a specific mRNA sequence. A cytosine right in between both proteins is base-specifically recognized by both proteins. Despite important protein-protein interactions in the RNA bound state, both proteins do not interact in absence of RNA. Several non-canonical RNA binding modes can be discerned. More details can be found in (Hennig et al., Nature, 2014).
Why is this important for humanity?
The RNA-binding protein is highly expressed in several cancer lines, foremost in melanoma (skin cancer) cells. We hypothesize that Unr has similar roles in translation regulation and could thus be a therapeutic target. We have initiated research into human Unr. The quest for a direct Sxl orthologue in humans has begun.
RNA-binding TRIM E3 ligases
TRIM proteins all share the conserved tripartite motif at the N-terminal region: a RING domain, conveying E3 ligase activity within the ubiquitin system, one or two B-box domains, followed by a coiled-coil domain for dimerization. The C-terminal region can feature a variety of domains and mediates ubiquitination substrate specificity. The PRYSPRY domain is most common (Figure 4A). Some TRIM proteins have been reported to bind RNA and we want to understand the link between RNA binding and ubiquitination. Does RNA mediate substrate proximity or does it regulate the ubiquitination activity of TRIM proteins? To answer these questions, we look specifically into the structure-function relationship of TRIM2, TRIM3 (both TRIM-NHL subfamily) and TRIM25 (TRIM-PRYSPRY subfamily).
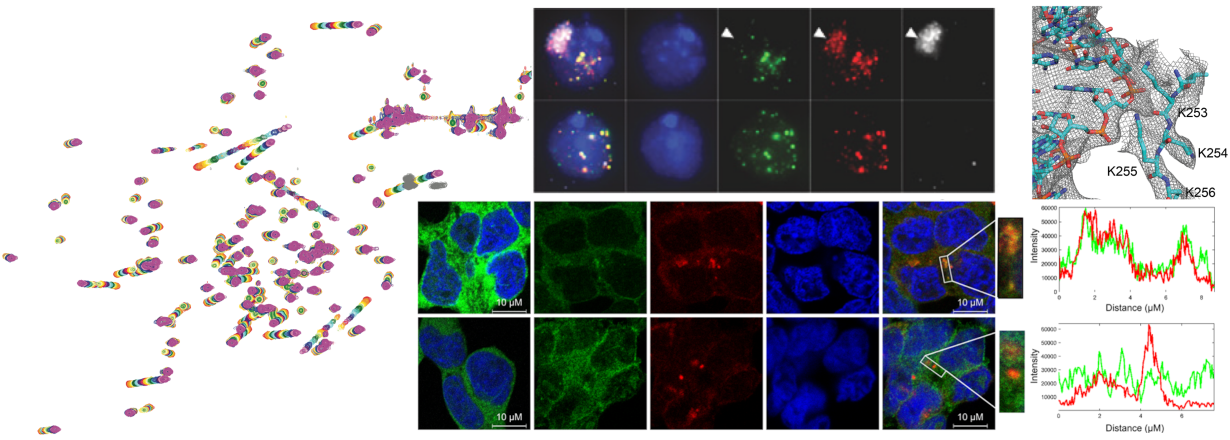
A key objective of our TRIM research is to unravel the RNA-dependent mechanisms of TRIM25’s antiviral activity. We could show that RNA binding is essential for slowing down viral replication in human cells (Figure 4B, unpublished). Our approach combines the precision of structural biology and biophysics with the practical insights of cell biology and viral infection experiments. Our work is further enriched by robust multinational collaborations, notably with the MRC-Centre for Virus Research in Glasgow. Such partnerships provide Hennig's team with invaluable, direct insights into the intricate molecular interactions between host and pathogen.
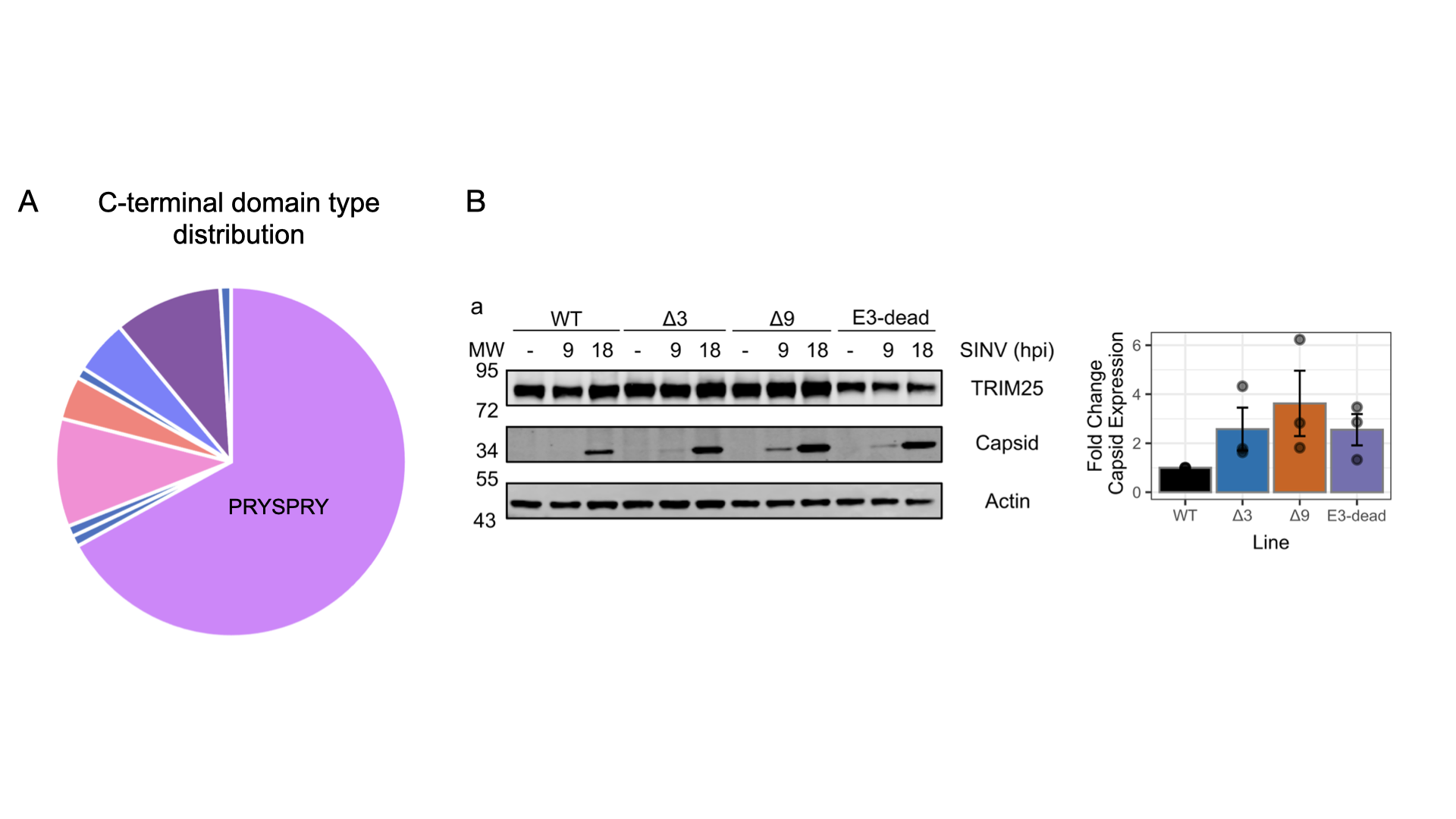
Figure 4: TRIM E3 ligases. A: The C-terminal region can consist of different domain types, with the majority of family members featuring the PRYSPRY domain. B: TRIM-PRYSPRY subfamily member TRIM25 is an RNA-binding TRIM protein and its antiviral activity depends on RNA binding. The Δ9 mutant, which is RNA binding deficient allows faster viral replication in HEK293 cells of Sindbis Virus (SINV).
Other RNA-related projects
The Hennig group is also involved in many collaborative project. Many of them RNA-related, e.g. understanding the basis of Tropomyosin-regulated RNA transport, riboregulation and many more. Contact us if you want to know more or see our publications: Hennig group’s publication list on PubMed